物理代写|热力学代写thermodynamics代考|EGR360
如果你也在 怎样代写热力学Thermodynamics 这个学科遇到相关的难题,请随时右上角联系我们的24/7代写客服。热力学Thermodynamics和宇宙本身一样古老,宇宙是已知的最大的热力学系统。当宇宙在呜咽中结束,宇宙的总能量消散为虚无时,热力学也将结束。
热力学Thermodynamics广义地说,热力学就是关于能量的:能量如何被利用,以及能量如何从一种形式转变为另一种形式。在很多情况下,热力学包括利用热做功,就像你的汽车发动机,或者做功来传递热量,就像你的冰箱。有了热力学,你就能知道事物如何有效地将能量用于有用的目的,比如移动飞机、发电,甚至骑自行车。
statistics-lab™ 为您的留学生涯保驾护航 在代写热力学thermodynamics方面已经树立了自己的口碑, 保证靠谱, 高质且原创的统计Statistics代写服务。我们的专家在代写热力学thermodynamics代写方面经验极为丰富,各种代写热力学thermodynamics相关的作业也就用不着说。
物理代写|热力学代写thermodynamics代考|The Molecular Partition Function
As might be expected from the previous section, corrected M-B statistics also leads to a unified expression for the most probable particle distribution. Indeed, if we invoke $g_j \gg N_j$, Eq. (4.2) becomes
$$
N_j=g_j \exp \left(\frac{\mu-\varepsilon_j}{k T}\right) .
$$
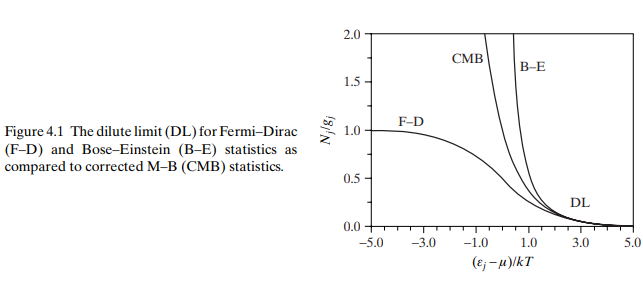
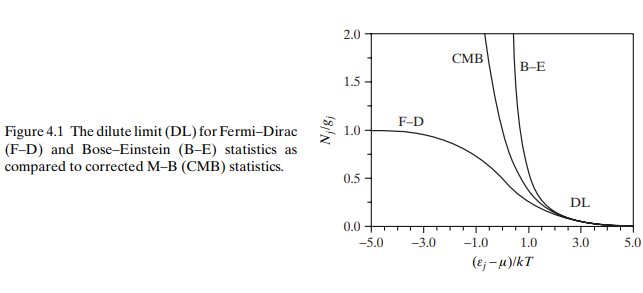
Equation (4.9) represents the equilibrium distribution in the dilute limit, as portrayed by Fig. 4.1 through comparisons with Eq. (4.2) for both B-E and F-D statistics. Notice that the $\mathrm{B}-\mathrm{E}$ and $\mathrm{F}-\mathrm{D}$ cases are equivalent and thus $g_j \gg N_j$ only when $\varepsilon_j \gg \mu$. Because $\varepsilon_j$ is
always positive, the dilute condition clearly applies when $\mu<0$. Such negative chemical potentials are characteristic of ideal gases.
Given Eq. (4.9), we next explore some important features of the equilibrium particle distribution for corrected M-B statistics. In particular, we may write
$$
N_j \exp \left(-\frac{\mu}{k T}\right)=g_j \exp \left(-\frac{\varepsilon_j}{k T}\right) .
$$
Now, summing over all $j$, from Eq. (4.10) we obtain
$$
N e^{-\mu / k T}=Z,
$$
where we have defined the dimensionless molecular partition function,
$$
Z=\sum_j g_j e^{-\varepsilon_j / k T} .
$$
物理代写|热力学代写thermodynamics代考|The Influence of Temperature
The importance of temperature to the equilibrium particle distribution can be further explored for two energy levels by casting Eq. (4.15) in the form
$$
\ln \left(\frac{g_k N_j}{g_j N_k}\right)=-\frac{\varepsilon_j-\varepsilon_k}{k T},
$$
where $\left(\varepsilon_j-\varepsilon_k\right) \geq 0$. Hence, at thermal equilibrium,
$$
\lim {T \rightarrow 0}\left(\frac{g_k N_j}{g_j N_k}\right)=0 \quad \lim {T \rightarrow \infty}\left(\frac{g_k N_j}{g_j N_k}\right)=1,
$$
so that, as temperature rises, the population shifts dramatically from predominance within lower energy levels to randomization among all energy levels. In other words, a greater temperature means enhanced particle energy and thus, from Eq. (4.16), more access to higher energy levels, as shown schematically in Fig. 4.2.
Continuing our thermal analysis, consider next the application of Eq. (4.15) to two energy levels, each containing a single energy state. If the energy of the ground state is $\varepsilon_0$, the population in the $i$ th energy state, when fractionally compared to that in the ground state, is
$$
\frac{N_i}{N_{\circ}}=\exp \left(-\frac{\varepsilon_i-\varepsilon_{\circ}}{k T}\right),
$$
where $\left(\varepsilon_i-\varepsilon_{\circ}\right) \geq 0$. Hence, at thermodynamic equilibrium, the population of any upper or excited energy state can never exceed that of the ground state. Consequently, a so-called population inversion is always indicative of a nonequilibrium process. Subsequent relaxation to the equilibrium distribution predicted by Eq. (4.14) invariably occurs through removal of excess energy from higher energy states via collisions or radiation, as discussed further in Chapter 11.
Presuming thermal equilibrium, we observe from Eqs. (3.14) and (4.17) that, as $T \rightarrow 0$, $E \rightarrow N \varepsilon_0$ because all particles following corrected M-B statistics are required to be in their ground state at absolute zero. Hence, a value of $\varepsilon_0$ must be specified to evaluate any properties involving the internal energy. Most often, we stipulate $\varepsilon_0=0$, which is equivalent to making all energy calculations relative to a zero of energy at absolute zero. This methodology ensures consistency with measured values of energy, which must also be taken relative to some arbitrary zero of energy. A similar procedure can, in fact, be used for bosons, fermions, or boltzons so that, in general, $U=0$ at $T=0$. In Chapter 13, we will show that the thermodynamic probability of a perfect crystalline solid is unity at absolute zero. Hence, from Eq. (3.19), we may also assume that $S=0$ at $T=0$. On this basis, all thermodynamic properties should vanish at absolute zero.
热力学代写
物理代写|热力学代写thermodynamics代考|The Molecular Partition Function
正如前一节所期望的那样,修正的M-B统计量也导致了最可能粒子分布的统一表达式。实际上,如果我们调用$g_j \gg N_j$, Eq.(4.2)变成
$$
N_j=g_j \exp \left(\frac{\mu-\varepsilon_j}{k T}\right) .
$$
式(4.9)表示稀释极限的平衡分布,通过与式(4.2)比较B-E和F-D统计量如图4.1所示。注意$\mathrm{B}-\mathrm{E}$和$\mathrm{F}-\mathrm{D}$的情况是等价的,因此只有在$\varepsilon_j \gg \mu$时才使用$g_j \gg N_j$。因为$\varepsilon_j$是
总是正的,稀释条件显然适用于$\mu<0$。这种负的化学势是理想气体的特征。
给定Eq.(4.9),我们接下来探讨修正M-B统计量的平衡粒子分布的一些重要特征。特别是,我们可以写
$$
N_j \exp \left(-\frac{\mu}{k T}\right)=g_j \exp \left(-\frac{\varepsilon_j}{k T}\right) .
$$
现在,把所有$j$加起来,从式(4.10)中我们得到
$$
N e^{-\mu / k T}=Z,
$$
我们定义了无量纲分子配分函数,
$$
Z=\sum_j g_j e^{-\varepsilon_j / k T} .
$$
物理代写|热力学代写thermodynamics代考|The Influence of Temperature
通过将式(4.15)代入式中,可以进一步探讨温度对两个能级的平衡粒子分布的重要性
$$
\ln \left(\frac{g_k N_j}{g_j N_k}\right)=-\frac{\varepsilon_j-\varepsilon_k}{k T},
$$
在哪里$\left(\varepsilon_j-\varepsilon_k\right) \geq 0$。因此,在热平衡时,
$$
\lim {T \rightarrow 0}\left(\frac{g_k N_j}{g_j N_k}\right)=0 \quad \lim {T \rightarrow \infty}\left(\frac{g_k N_j}{g_j N_k}\right)=1,
$$
因此,随着温度的升高,种群从低能级的优势急剧转变为所有能级的随机化。换句话说,更高的温度意味着粒子能量的增强,因此,从式(4.16)可以看出,更多的粒子进入更高的能级,如图4.2所示。
继续我们的热分析,接下来考虑将式(4.15)应用于两个能级,每个能级包含一个能态。如果基态的能量为$\varepsilon_0$,那么与基态相比,$i$第1个能态的占比为
$$
\frac{N_i}{N_{\circ}}=\exp \left(-\frac{\varepsilon_i-\varepsilon_{\circ}}{k T}\right),
$$
在哪里$\left(\varepsilon_i-\varepsilon_{\circ}\right) \geq 0$。因此,在热力学平衡时,任何高能级或激发态的占比永远不会超过基态的占比。因此,所谓的人口反转总是表明一个非平衡过程。由式(4.14)预测的平衡分布的后续松弛总是通过碰撞或辐射从高能态去除多余能量而发生的,如第11章进一步讨论的那样。
假设热平衡,我们从式中观察到。(3.14)和(4.17),即$T \rightarrow 0$, $E \rightarrow N \varepsilon_0$,因为所有遵循修正M-B统计量的粒子都需要处于绝对零度的基态。因此,必须指定$\varepsilon_0$值来评估涉及内能的任何属性。大多数情况下,我们规定$\varepsilon_0=0$,这相当于在绝对零度下进行所有能量计算。这种方法确保了与能量测量值的一致性,这些测量值也必须相对于某个任意的能量零来取。事实上,类似的程序可以用于玻色子、费米子或玻色子,因此,一般来说,$U=0$在$T=0$。在第13章中,我们将证明完美结晶固体的热力学概率在绝对零度是统一的。因此,从式(3.19)中,我们也可以假设$S=0$ = $T=0$。在此基础上,所有的热力学性质都应该在绝对零度消失。
统计代写请认准statistics-lab™. statistics-lab™为您的留学生涯保驾护航。
金融工程代写
金融工程是使用数学技术来解决金融问题。金融工程使用计算机科学、统计学、经济学和应用数学领域的工具和知识来解决当前的金融问题,以及设计新的和创新的金融产品。
非参数统计代写
非参数统计指的是一种统计方法,其中不假设数据来自于由少数参数决定的规定模型;这种模型的例子包括正态分布模型和线性回归模型。
广义线性模型代考
广义线性模型(GLM)归属统计学领域,是一种应用灵活的线性回归模型。该模型允许因变量的偏差分布有除了正态分布之外的其它分布。
术语 广义线性模型(GLM)通常是指给定连续和/或分类预测因素的连续响应变量的常规线性回归模型。它包括多元线性回归,以及方差分析和方差分析(仅含固定效应)。
有限元方法代写
有限元方法(FEM)是一种流行的方法,用于数值解决工程和数学建模中出现的微分方程。典型的问题领域包括结构分析、传热、流体流动、质量运输和电磁势等传统领域。
有限元是一种通用的数值方法,用于解决两个或三个空间变量的偏微分方程(即一些边界值问题)。为了解决一个问题,有限元将一个大系统细分为更小、更简单的部分,称为有限元。这是通过在空间维度上的特定空间离散化来实现的,它是通过构建对象的网格来实现的:用于求解的数值域,它有有限数量的点。边界值问题的有限元方法表述最终导致一个代数方程组。该方法在域上对未知函数进行逼近。[1] 然后将模拟这些有限元的简单方程组合成一个更大的方程系统,以模拟整个问题。然后,有限元通过变化微积分使相关的误差函数最小化来逼近一个解决方案。
tatistics-lab作为专业的留学生服务机构,多年来已为美国、英国、加拿大、澳洲等留学热门地的学生提供专业的学术服务,包括但不限于Essay代写,Assignment代写,Dissertation代写,Report代写,小组作业代写,Proposal代写,Paper代写,Presentation代写,计算机作业代写,论文修改和润色,网课代做,exam代考等等。写作范围涵盖高中,本科,研究生等海外留学全阶段,辐射金融,经济学,会计学,审计学,管理学等全球99%专业科目。写作团队既有专业英语母语作者,也有海外名校硕博留学生,每位写作老师都拥有过硬的语言能力,专业的学科背景和学术写作经验。我们承诺100%原创,100%专业,100%准时,100%满意。
随机分析代写
随机微积分是数学的一个分支,对随机过程进行操作。它允许为随机过程的积分定义一个关于随机过程的一致的积分理论。这个领域是由日本数学家伊藤清在第二次世界大战期间创建并开始的。
时间序列分析代写
随机过程,是依赖于参数的一组随机变量的全体,参数通常是时间。 随机变量是随机现象的数量表现,其时间序列是一组按照时间发生先后顺序进行排列的数据点序列。通常一组时间序列的时间间隔为一恒定值(如1秒,5分钟,12小时,7天,1年),因此时间序列可以作为离散时间数据进行分析处理。研究时间序列数据的意义在于现实中,往往需要研究某个事物其随时间发展变化的规律。这就需要通过研究该事物过去发展的历史记录,以得到其自身发展的规律。
回归分析代写
多元回归分析渐进(Multiple Regression Analysis Asymptotics)属于计量经济学领域,主要是一种数学上的统计分析方法,可以分析复杂情况下各影响因素的数学关系,在自然科学、社会和经济学等多个领域内应用广泛。
MATLAB代写
MATLAB 是一种用于技术计算的高性能语言。它将计算、可视化和编程集成在一个易于使用的环境中,其中问题和解决方案以熟悉的数学符号表示。典型用途包括:数学和计算算法开发建模、仿真和原型制作数据分析、探索和可视化科学和工程图形应用程序开发,包括图形用户界面构建MATLAB 是一个交互式系统,其基本数据元素是一个不需要维度的数组。这使您可以解决许多技术计算问题,尤其是那些具有矩阵和向量公式的问题,而只需用 C 或 Fortran 等标量非交互式语言编写程序所需的时间的一小部分。MATLAB 名称代表矩阵实验室。MATLAB 最初的编写目的是提供对由 LINPACK 和 EISPACK 项目开发的矩阵软件的轻松访问,这两个项目共同代表了矩阵计算软件的最新技术。MATLAB 经过多年的发展,得到了许多用户的投入。在大学环境中,它是数学、工程和科学入门和高级课程的标准教学工具。在工业领域,MATLAB 是高效研究、开发和分析的首选工具。MATLAB 具有一系列称为工具箱的特定于应用程序的解决方案。对于大多数 MATLAB 用户来说非常重要,工具箱允许您学习和应用专业技术。工具箱是 MATLAB 函数(M 文件)的综合集合,可扩展 MATLAB 环境以解决特定类别的问题。可用工具箱的领域包括信号处理、控制系统、神经网络、模糊逻辑、小波、仿真等。